
Water Quality Terms
Lakes are a limited, non‑renewable resource. They are especially sensitive to human disturbance because they act as collecting basins for all the water which flows from the lands which surround them. As rainfall and snowmelt wash across the land’s surface, these forces lift loose soil, carry it along, and finally deposit it in tributary streams and lakes. This is harmful because every soil particle carries with it a nutrient for plants called phosphorus. When it gets into a lake, phosphorus feeds microscopic floating organisms called algae or cyanobacteria. If too much phosphorus is added, algal populations explode and lake water becomes cloudy, smelly, and uninviting.
Watershed — An area of land that drains water, sediment and dissolved materials to a common receiving body or outlet. The term is not restricted to surface water runoff and includes interactions with subsurface water. Watersheds vary from the largest river basins to just acres or less in size. In our area the relevant watershed is approximately 180 square miles that include parts of 13 communities, seven major lakes and several smaller water bodies. The outlet is the head of Messalonskee stream. To protect and preserve our lakes we must pay attention to the entire Belgrade Watershed: all lands and all waters.
Phosphorus loading refers to all processes which carry phosphorus to the lakes and streams of the watershed. In a state of nature, lakes remain sparkling and clear for tens or even hundreds of thousands of years because trees, grasses and bushes protect them from soil erosion and phosphorus loading. Human development speeds rainwater runoff and increases phosphorus loading by exposing soil, removing native cover, leveling the ground and covering it with non‑absorbent surfaces like buildings, roads, and parking lots.
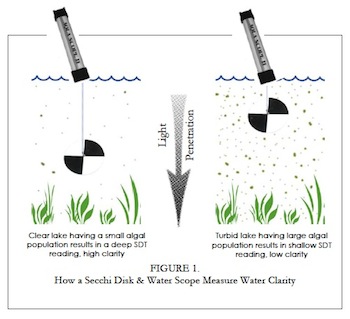 Secchi disk measurement
Secchi disk measurement
Water clarity is diminished as algae and cyanobacteria prosper from the long‑term increase in phosphorus. Clarity is expressed as “Secchi depth” which is the depth at which a special disc becomes invisible below the water surface. Long Pond’s long‑term decrease has caused the State to design a program to control phosphorus loading.
Gloeotrichia echinulata is an organism that forms tiny clusters in the lake water. It has been seen in our lakes at least since 1987, but wasn’t particularly noticeable until 2002. Since then, occasional phosphorus gushes have promoted episodes of Gloeotrichia overgrowth that collect on the surface as a green scum called a bloom.
Algae and blue‑green algae are outdated names for the group of organisms to which Gloeotrichia belongs: properly called cyanobacteria. Scientists recently changed the name to reflect new knowledge. However, the old terms are still often used. Please see GLOEOTRICHIA Frequently Asked Questions.
Remediation refers to fighting the blooms and water quality decline. The fight requires new funds. Remediation may target long‑term, widespread effects or localized, short‑term impacts. We have many long‑term programs underway already. The Belgrade Lakes Association advocates landscaping that filters rainwater and road maintenance that prevents runoff. We support the LakeSmart program for homeowners who want to learn how to improve lake water quality—or call the Youth Conservation Corps (207-495‑6039) which provides labor for runoff abatement. Two localized, short‑term methods—ultrasound and on‑shore pumping—are scheduled for test trials this summer. Ultrasound ideally will use high‑frequency sound waves to kill scum organisms with little or no other effects; pumping will remove scum from surface water and deposit it in uninhabited lakeshore areas.
For News, stay tuned to the Belgrade Lakes Association website: https://www.belgradelakesassociation.org/Resources/News-and-Announcements
Alkalinity
Alkalinity is the capacity of water to neutralize acid. Often confused with pH—which measures the strength of an acid or base—alkalinity is a measure of a solution’s ability to resist changes in pH. It’s frequently expressed as equivalent mg/L CaCO3. In natural waters, alkalinity is essentially equal to the sum of the molar quantities of carbonate, bicarbonate, and hydroxyl ions. Under certain conditions, borates, silicates and phosphates can also contribute to alkalinity.
The main source of alkalinity in natural waters is dissolution of carbonate rocks such as limestone. Streams in limestone regions tend to have high alkalinity and are resistant to pH changes, making them better able to buffer acid rain than granitic mountain lakes. Maintaining pH is important for fish and other aquatic organisms: if pH is lowered due to acid rain, heavy metals can leach from sediments, raising contaminant levels.
Alkalinity is measured in the laboratory by titration after converting carbonate and bicarbonate to carbonic acid by lowering the pH. Samples should be refrigerated and processed within 24 hours of collection.
Water Temperature Standards
| Period |
Maximum Temperature (°F) |
| Jan 1–31 |
40 |
| Feb 1–29 |
40 |
| Mar 1–31 |
46 |
| Dec 1–31 |
42 |